Transcutaneous Spinal Cord Stimulation: A New Hope for Spinal Injuries
Welcome to our latest exploration in the captivating realm of neurorehabilitation. Today, we venture into the innovative field of transcutaneous spinal cord stimulation (tSCS) - a groundbreaking approach that has been making waves in recent years. Like Functional Electrical Stimulation, Neurofeedback and biofeedback, tSCS is within the general category of applications being referred to as neuromodulation. Here is a brief introductory video
Essentially, tSCS is a non-invasive method that involves sending small electrical currents transcutaneously to stimulate the spinal cord. This means that the stimulation is delivered through the skin rather than by directly implanting electrodes into the spinal cord or the surrounding tissues. Particularly at this early stage of development, avoiding surgical implantation of a device seems sensible and low risk.
This technique is built on the idea that our bodies are marvelous conductive systems, capable of transmitting these electrical signals to aid in the reactivation of lost functions, particularly in people who have experienced spinal cord injuries. Spinal cord injuries can lead to a range of complications, including paralysis and loss of sensation, which can be life-altering.
As we all know, the spinal cord plays a vital role in transmitting sensory and motor signals between the brain and the body's organs and extremities. In cases of spinal cord injury, whether these injuries are classed as 'complete' or 'incomplete', the spinal cord's functionality can be severely impaired, leading to a loss of motor function, touch sensation, chronic pain, and other debilitating symptoms.
Research has been underway to utilise tSCS in rehabilitating individuals with spinal cord injuries, opening new doors to hope and recovery. By the end of this blog post, we aim to enlighten you about the science behind tSCS, and the potential it holds for improving the quality of life in spinal cord injury survivors.
How it began
Research has demonstrated that animals with completely severed spinal cords could walk proficiently on a treadmill with partial body weight support [see Articles 13 - 14]. The theory behind this research, which relied on the presence of so-called central pattern generators, was that the movement of the legs by the treadmill would activate sensory afferents in the limbs that in turn switched on these spinal generators.
This research then explored several non-invasive forms of stimulus energy, such as electrical, magnetic and vibration, and found that they could, for example, augment the effects of treadmill training. One approach was referred to as transcutaneous spinal cord stimulation.
This approach has been shown since 1982 to enhance the excitability of spinal neural circuits, which has the potential to improve voluntary performance in people with incomplete injuries [Article 15].
This research suggests that this change in excitability enables the brain to utilise functionally silent descending pathways to produce and enhance voluntary movements. In other words, it facilitates neuroplasticity.
It is now thought that the priming of the nervous system offered via tSCS could be used to augment existing physical rehabilitation interventions such as exoskeleton-assisted walking, sit-to-stand exercises, FES Cycling and more.
Stimulation settings
Electrical stimulation for the lower limbs is typically delivered with skin surface electrodes placed to support the goals of the exercise as we will reveal below. A biphasic rectangular waveform with 1-millisecond pulse width and a frequency of 5–50 Hz seems to be typical. Some studies have used a higher frequency carrier signal, but this doesn’t seem to be necessary; it appears that a readily available biphasic waveform generator will potentially do the job.
The stimulation current is typically 20–80 mA and adjusted until the participant reports tingling in their extremities. Once the parameters are set, the participant can carry out the activity-based training.
There is a general lack of consensus at the moment on these stimulation parameters, which may be one reason why this approach has not had greater clinical deployment up until now. Exclusion criteria are generally the same as with other electrical stimulation applications, including open wounds at the stimulation site, pregnancy, active cancer, presence of a cardiac pacemaker/defibrillator or uncontrolled autonomic dysreflexia.
There are many unknowns about this technique and we do not yet understand if the changes associated with it are long-lasting, need to be refreshed from time to time or whether tSCS should be considered like an orthosis and be present all the time. Despite this, the results in support of tSCS are compelling. It is low-risk and easy to apply as an adjunct to training methods.
Where on the body has tSCS been applied and why
As mentioned above, tSCS has been applied to various regions of the spinal cord, each with a distinct purpose depending on the area stimulated. It can also be used alone or in combination with other therapies to enhance the overall treatment outcomes.
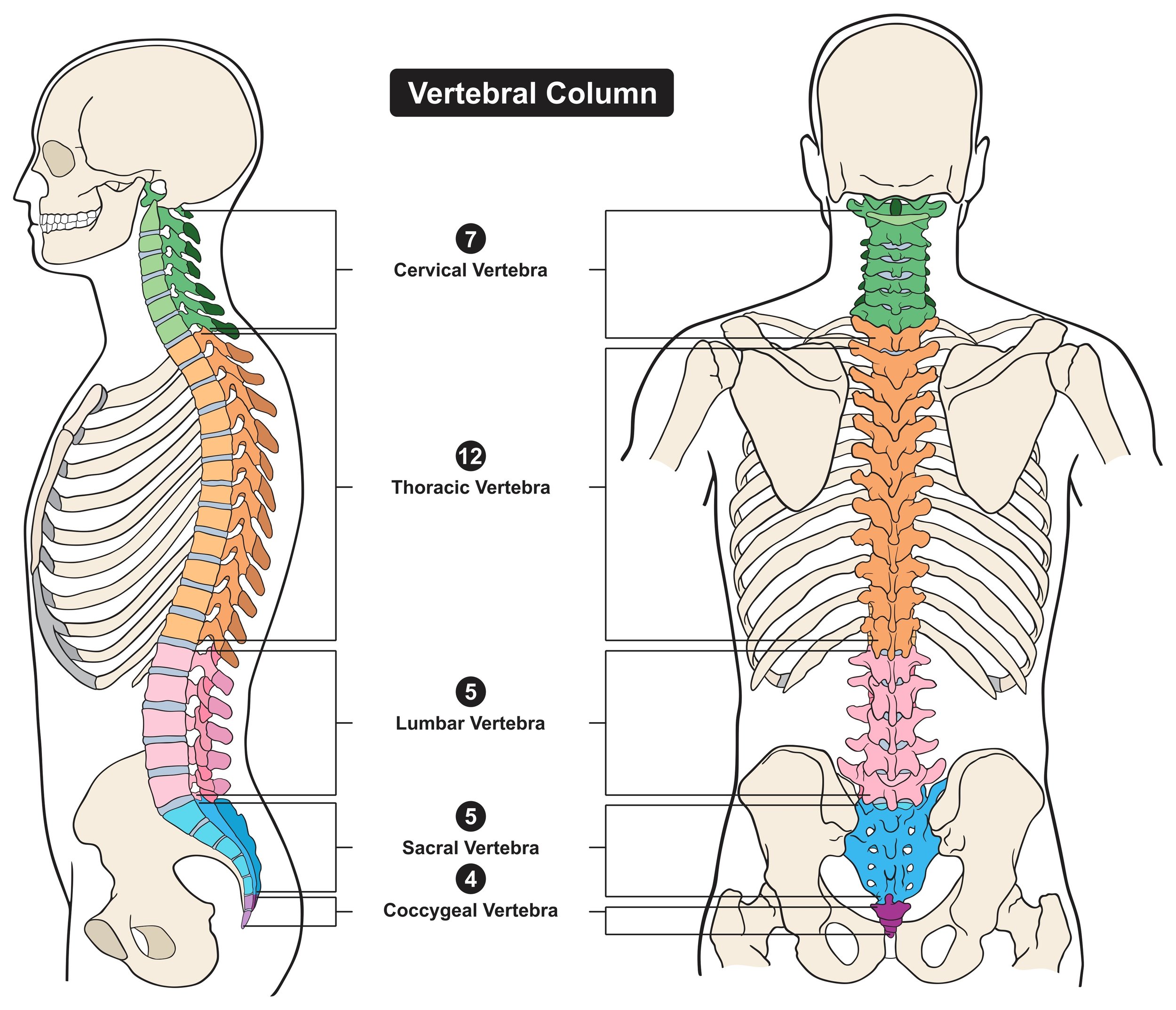
Cervical region: The cervical region of the spinal cord is located in the neck and corresponds to the functions of the arms and hands. Stimulating this region can potentially aid in improving hand and arm functions, which are crucial for daily living activities.
Thoracic region: This part of the spinal cord, located in the chest area, controls some of the muscles of the torso. Whilst not traditionally targeted for movement recovery, stimulation here may help with postural stability and balance and potentially could impact autonomic functions like cardiovascular and respiratory control. (See articles 1 - 4)
Lumbosacral region: The lumbosacral region is at the lower end of the spinal cord, corresponding to the function of the legs and pelvic organs. Stimulating this region aims to restore leg functions, including standing and walking. Additionally, it can potentially help with bladder, bowel, and sexual function - all of which can be significantly affected by spinal cord injuries. tSCS stimulation over the thoracolumbar region at moderate frequencies (5–40Hz) generates locomotor-like muscle activity and movements in paralysed or paretic muscles after spinal cord injury [Articles 5 - 8]. tSCS may be used to augment the benefits of locomotor training although studies incorporating combined interventions are rare at the moment. (Article 9). tSCS has also been found to reduce spasticity with an effect that persists once the stimulation is turned off [Article 10 and Article 12],
Research is ongoing and the boundaries of what we understand are continually expanding. Certainly, we are certain that different regions can be stimulated for various outcomes, and there is ongoing research into the best stimulation parameters to use - the frequencies, intensities, and timings for these stimulations. As our knowledge in this area grows, researchers hope to improve their methods and offer more precise, individualised treatments to those affected by spinal cord injuries.
A novel example of combining tSCS with functional electrical stimulation
Wiesener et al [Article 11] describe a hybrid exercise modality that combines functional electrical stimulation (FES) of the knee extensors with tSCS in paraplegic front crawl swimming. in this novel application, tSCS is used to stimulate the afferent fibres of the L2–S2 posterior roots for spasticity reduction. Stimulation was applied continuously at 50Hz using biphasic pulses with 1 ms pulse width over the T11/12 region at the spinal cord according to the process in Article 12.
From Article 11 - Utising tSCS and FES to facilitate paraplegic swimming
By activating the tSCS, the trunk musculature was recruited at a motor level. This aims to improve trunk stability and straighten the upper body.
Within the feasibility study, two complete spinal cord injured subjects (both ASIA scale A, lesion level Th5/6), who have previously been proficient front crawl swimmers, conducted a 10-week swim training with stimulation support.
In an additional assessment, nine months after the training, the knee extension, hip extension, and trunk roll angles were measured using waterproof inertial measurement units and compared for different swimming conditions (no stimulation, tSCS alone, FES alone, FES plus tSCS).
For both subjects, a positive training effect was noted over the 10-week swim training in terms of measured lap times (16 m pool) for all swimming conditions. Swimming supported by FES reduced lap times by 15.4% and 8.7% on average for Subject A and Subject B, respectively. Adding tSCS support yielded even greater mean decreases of 19.3% and 20.9% for Subjects A and B, respectively. Additionally, both subjects individually reported that swimming with tSCS for 30–45 minutes eliminated spasticity in the lower extremities for up to 4 hours beyond the duration of the session. Both subjects reported that stimulation-assisted swimming is comfortable and enjoyable, and they would like to use such a device for recreational training and rehabilitation in the future.
Research studies closer to home
Spinal Research charity (https://spinal-research.org/about-us) is currently funding three UK studies applying tSCS.
Dr Ronaldo Ichiyama and Sarah Astill (Leeds University): are examining tSCS for recovery of arm and hand function in individuals with cervical spinal cord injury.
This project builds on a pilot study which proved the safety, tolerability and feasibility of a novel non-invasive transcutaneous spinal stimulation device. The present project will test the efficacy of the treatment on patients with chronic spinal cord injury - so that any improvements can be ascribed solely to the treatment. The team will be assessing whether using tSCS in conjunction with intense periods of task-specific training could lead to the progression along the American Spinal Injuries Association (ASIA) scale.
Dr Mariel Purcell and Dr Aleksandra Vuckovic (Glasgow University) are determining the effects of tSCS on sensorimotor function in chronic complete tetraplegia. This study is also expanding on a previous pilot study that demonstrated that tSCS shows promise in restoring function. To expand on the study effectively, the team in Glasgow are using a larger patient cohort, involving non-ventilator-dependent tetraplegics injured at the C2-C6 level. In combination with functional activity-based exercise training, participants will undergo weeks of personalised neuromodulation. This study aims to determine the immediate and lasting effects of these interventions on motor, sensory and autonomic function as well as identify an effective dose and duration of neuromodulation needed to reveal and maintain any latent function.
Jane Symonds (Neurokinex Charitable Trust) is looking at non-invasive spinal cord stimulation combined with activity-based rehabilitation in chronic spinal cord injury. Unlike the previous two studies, this third project will take place in a specialised rehabilitation centre. Neurokinex, a charity providing specialised neurological rehabilitation for various forms of paralysis, will recruit patients with either neck or back injuries and provide 12 months of rehabilitative intervention to determine the long-term effects of this novel treatment. This arm of the package of studies involving neuromodulation will provide valuable information on the effects on a lower limb as well as upper limb function and the potential for benefits in the bladder, bowel and cardiovascular systems.
Conclusion
In conclusion, transcutaneous spinal cord stimulation provides an effective and non-invasive method to promote pain relief, improve motor function, and enhance the overall quality of life for patients with spinal cord injuries. By using electrical stimulation and modulating spinal excitability, tSCS has the potential to significantly contribute to rehabilitation potential and quality of life. tSCS offers a promising avenue for patients to regain voluntary movement, reduce spasticity, and improve their overall well-being. It is important to note that tSCS is not limited to spinal cord injury but can also be utilized in individuals with other neurological disorders that affect the spinal cord.
As always with research, much more needs to be done. In this case, we need to explore how to optimise the stimulation parameters and understand whether the effects persist or need to be applied for the longer term. As the approach is relatively safe and results have been encouraging it is hoped that this can accelerate the deployment of these techniques in practice.
Articles related to tSCS
1) Gad PN, Kreydin E, Zhong H, Latack K, Reggie Edgerton V. Non-invasive neuro-modulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci. 2018;1:432.
2) Kreydin E, Zhong H, Latack K, Ye S, Edgerton VR, Gad P. Transcutaneous electrical spinal cord neuromodulator (TESCoN) improves symptoms of overactive bladder. Front Syst Neurosci. 2020;14:1.
3) Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma. 2018;35:446–51.
4) Harkema SJ, Wang S, Angeli CA, Chen Y, Boakye M, Ugiliweneza B. et al. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front Hum Neurosci. 2018;12:83.
5) Hofstoetter US, Krenn M, Danner SM, Hofer C, Kern H, McKay WB, et al. Aug- mentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39: E176–86.
6) Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, et al. Noninvasive reactivation of motor descending control after paralysis. J Neuro- trauma. 2015;32:1968–80.
7) Gerasimenko Y, Sayenko D, Gad P, Kozesnik J, Moshonkina T, Grishin A, et al. Electrical spinal stimulation, and imagining of lower limb movements to modulate brain-spinal connectomes that control locomotor-like behavior. Front Physiol. 2018;9:1196.
8) Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, et al. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol. 2015;113:834–42.
9) Zaaya M, Pulverenti TS, Knikou M. Transspinal stimulation and step training alter function of spinal networks in complete spinal cord injury. Spinal Cord Ser Cases. 2021 Jul 3;7(1):55. doi: 10.1038/s41394-021-00421-6. PMID: 34218255; PMCID: PMC8254806.
10) Hofstoetter US, Freundl B, Danner SM, Krenn MJ, Mayr W, Binder H, et al. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J Neurotrauma. 2020;37:481–93.
11) Wiesener C, Spieker L, Axelgaard J, Horton R, Niedeggen A, Wenger N, Seel T, Schauer T. Supporting front crawl swimming in paraplegics using electrical stimulation: a feasibility study. J Neuroeng Rehabil. 2020 Apr 16;17(1):51. doi: 10.1186/s12984-020-00682-6. PMID: 32299483; PMCID: PMC7164248.
12) Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37(2):202–11.
13) Forssberg, H (1980a) ‘The locomotion of the low spinal cat I. Coordination within a hindlimb’, Acta Physiologica Scandinavica, 108, pp269–281
14) Forssberg, H (1980b) ‘The locomotion of the low spinal cat II. Interlimb coordination’, Acta Physiologica Scandinavica, 108, pp283–295
15) Martin, R (2021) ‘Utility and feasibility of transcu- taneous spinal cord stimulation for patients with incomplete SCI in therapeutic settings: A review of the topic’, Frontiers in Rehabilitation Sciences, 2, www. frontiersin.org/articles/10.3389/fresc.2021.724003/ full