FES Cycling and cardiovascular fitness following a spinal cord injury?
Introduction
The effects of a spinal cord injury (SCI) vary depending on the neurological level of the damage and the extent of neural traffic interruption at the site of the lesion. Common primary effects may include paralysis and loss of sensation in the legs, arms, and trunk, as well as disruptions in bladder and bowel function and the regulation of blood pressure, heart rate, and lung function.
Muscle paralysis and the resulting reduction in effective muscle mass can hinder individuals from effectively exercising to maintain their cardiovascular fitness. This, in turn, may increase the risk of heart disease or metabolic disease as a secondary consequence of the spinal injury. Exercise capacity of individuals with an SCI is inversely related to spinal lesion level.
We all know that effective cardiovascular function is essential to health and involves the heart, but we probably don't tend to give as much attention to the "vascular" part of the circulatory system. However, health is not just about the heart as a pump. The status of the whole dynamic system and vascular adaptations are vital for health. By influencing the blood vessels (the body's "plumbing") and making them constrict or dilate, the limited amount of blood that circulates in our bodies can be diverted to where it is needed most.
A spinal cord injury leads to many adaptations that influence rehabilitation and long-term health. In this article, we will look at this topic in detail and how FES Cycling can help persons following a spinal cord injury maintain fitness with a particular focus on cardiovascular function.
Adaptations
A spinal cord injury causes disruptions to cardiovascular function. Vascular adaptations are of paramount importance when it comes to understanding the complex relationship between an SCI and the subsequent recovery process. An injured spinal cord triggers a complex series of physiological changes within the body, and these adaptations in the vascular system are instrumental in supporting the healing journey. They involve a delicate interplay between blood vessels, autonomic nervous system function, and cardiovascular responses, all working together to facilitate rehabilitation and restoration.
One crucial facet of vascular adaptations following an SCI is thought to be spinal cord perfusion. When the spinal cord sustains an injury, normal blood flow to this vital structure is compromised, resulting in reduced perfusion. Consequently, the body launches a concerted effort to restore and optimise blood flow to the injured area. The key to this is the increase in vascular tone. Vascular resistance is a mechanism to maintain an adequate supply of oxygenated blood and essential nutrients to the damaged tissue, thereby promoting healing.
As we stated earlier, cardiovascular function is significantly affected by the level of SCI.
Individuals with a cervical-level SCI, for example, may experience a reduction in cardiac output. This reduction arises due to both impaired muscle function and decreased physical activity. Unfortunately, a decrease in cardiac output can have far-reaching ramifications on cardiovascular health, thereby increasing the likelihood of developing associated conditions such as hypertension and stroke in the longer term.
Research suggests (Maria T. E. Hopman 2002) that SCI individuals' leg vascular resistance is dramatically increased. This may be caused by structural (a decrease in the number of arterioles and capillaries and/or a decrease in the diameter of the resistance vessels) and functional changes (changes in endothelium-derived factors and/or sympathetic vascular regulation). The increased leg vascular resistance is reversible toward normal values by training the paralysed legs with electrical stimulation of the muscle. FES Cycling provides an opportunity to actively exercise paralysed leg muscles, with one aim being normalising vascular resistance.
The cardiovascular system
Here is a very basic rundown of how the system works. The cardiovascular system, comprising the heart, arteries, veins, and capillaries, is essential for transporting blood throughout the body, supplying oxygen and nutrients and removing the waste products of metabolism. The vascular component, including arteries and veins, is crucial in moderating blood flow, especially during exercise and, as mentioned above, in response to trauma.
The body has to be able to arrange for a necessary and sufficient amount of blood to be directed to where it is needed from moment to moment. This is achieved with a complex control system that usually allows the body to adapt to its demands automatically.
1. Arteries and Exercise:
Vasodilation: During exercise, the muscles require more oxygen and nutrients to carry out work demands (above the demands for staying alive). This demand leads typically to the vasodilation (widening) of arteries and arterioles (small arteries) supplying the active muscles. Vasodilation is primarily mediated by the release of nitric oxide (NO) from the endothelial cells lining the blood vessels, which relaxes the smooth muscle cells in the vessel walls.
Increased Blood Flow: Vasodilation increases the diameter of the blood vessels, reducing vascular resistance. This allows a greater blood volume to flow through the vessels, enhancing the supply of oxygen and nutrients to the working muscles.
Blood Pressure Regulation: Despite the increased blood flow, the body regulates blood pressure through various mechanisms, including the autonomic nervous system, which adjusts heart rate and the force of heart contractions.
Although the typical response to the demands of exercise involves vasodilation of the involved muscles, we now know that vasoconstriction can actually occur under intense exercise. There is a "sweet spot" in which the blood flow to a muscle is optimised. The key seems to be to favour the volume of exercise carried out rather than absolute intensity.
Based on findings from supervised exercise trials in controlled laboratory settings, physical activity guidelines have recently been developed for the SCI population. (Hoekstra et al 2020) These guidelines recommend at least 20 minutes of moderate-to-vigorous aerobic activity twice weekly, complemented with resistance exercise for fitness benefits, and at least 30 minutes of moderate-to-vigorous aerobic activity three times per week for cardiometabolic benefits.
2. Veins and Exercise:
Venous Return: Exercise affects the veins, which carry deoxygenated blood back to the heart. Muscle contractions during physical activity compress the veins, acting as a pump to help return blood to the heart, a mechanism known as the muscle pump.
Vasoconstriction blood backflow increases: Veins can constrict under sympathetic nervous system control, increasing venous return to the heart during exercise.
Valves in Veins: Veins have one-way valves that prevent blood backflow, ensuring it moves in the right direction back to the heart. Combined with these valves, the muscle pump mechanism efficiently aids venous return during physical activity.
Following an SCI resulting in lower limb paralysis, venous return is compromised due to the loss of normal muscle contractions.
3. Adaptation to Exercise:
Capillary Density: Regular exercise of sufficient amount and intensity can increase capillary density around the muscles, improving the blood's ability to deliver oxygen and nutrients.
Arteriogenesis and Angiogenesis: Long-term physical activity of sufficient amount and intensity can lead to the development of new blood vessels (angiogenesis) and the enlargement of existing ones (arteriogenesis), further improving blood supply to muscles during exercise.
4. Blood Flow Regulation Mechanisms:
Local Factors: Increased carbon dioxide (CO2) levels, lower pH, and higher temperatures in active muscles signal the need for increased blood flow.
Neural Control: The autonomic nervous system adjusts the diameter of blood vessels and heart function in response to exercise.
Hormonal Control: Hormones like adrenaline (epinephrine) can influence heart rate, force of contraction, and blood vessel diameter, affecting blood flow during exercise.
In summary, the cardiovascular system adapts dynamically to the demands of exercise through a complex interplay of local, neural, and hormonal signals that modulate blood vessel diameter, blood flow, and heart function. This ensures muscles receive the necessary oxygen and nutrients for sustained physical activity while maintaining overall circulatory health.
Cardiovascular Adaptations Following a Spinal Cord Injury
Following a spinal cord injury, significant adaptations occur within the cardiovascular system, primarily due to the disruption of the autonomic nervous system pathways that control heart rate, blood pressure, and blood flow distribution. These changes can lead to a range of cardiovascular complications and require careful management. Here are some of the adaptations and challenges and how the level of injury influences these.
1. Impaired Autonomic Control:
Autonomic Dysreflexia: Individuals with an SCI, especially those above the T6 level, may experience autonomic dysreflexia. This is an intense and potentially dangerous condition characterised by an unchecked and excessive sympathetic nervous system response, resulting in a rapid spike in blood pressure. While autonomic dysreflexia can be alarming, it aims to ensure that the spinal cord and other critical organs obtain the blood flow and oxygenation necessary for optimal functioning.
Orthostatic Hypotension: The loss of sympathetic control often leads to a decrease in blood pressure upon standing (orthostatic hypotension), as the normal vasoconstriction that would counteract gravity's effect on blood pooling in the legs is impaired. This can cause dizziness, lightheadedness, or even fainting upon changing positions.
2. Cardiovascular Function Adjustments:
Decreased Heart Rate Response: SCI can lead to a "blunted" heart rate response to exercise or stress, particularly in injuries above the T1 level, where the sympathetic innervation to the heart is disrupted. This makes it challenging to increase cardiac output during physical activity adequately.
Reduced Stroke Volume: The ability of the heart to increase stroke volume (the amount of blood ejected with each heartbeat) may be limited, affecting overall cardiovascular capacity and endurance.
3. Venous Return Challenges:
Reduced Muscle Pump Activity: The loss of mobility and muscle function, especially in the lower limbs, decreases the effectiveness of the muscle pump, leading to reduced venous return. This can increase the risk of venous stasis and deep vein thrombosis (DVT).
Changes in Blood Volume Distribution: SCI can alter blood volume distribution, with more blood pooling in the extremities due to reduced venous return. This can further complicate cardiovascular regulation.
As we will see, FES Cycling is one way we can help venous return challenges by actively encouraging the muscles to contract and relax - the muscle pump is active.
4. Adaptive Mechanisms:
Compensatory Sweating: In cases of high-level SCI, the body may adapt by increasing sweating below the level of injury to regulate blood pressure and temperature despite losing conscious control over these areas.
Blood Vessel Sensitivity Changes: There can be an increase in the sensitivity of peripheral blood vessels to circulating catecholamines, partially compensating for the loss of direct sympathetic control over vasoconstriction.
5. Long-term Adaptations:
Physical Conditioning: Engaging in adaptive physical activities and exercises designed for individuals with SCI can help improve cardiovascular function, enhance venous return, and increase overall physical health. Such programs often include using FES Cycling and adaptive equipment to facilitate cardio exercises.
Pharmacological Interventions: Medications may be used to manage blood pressure, prevent DVT, and address other cardiovascular concerns specific to the individual's condition and level of SCI.
These adaptations and related challenges highlight the complexity of managing cardiovascular health following SCI. A multidisciplinary approach, including physiotherapy and specialised medical care, is essential for optimising cardiovascular function, preventing complications, and improving the quality of life for individuals with spinal cord injuries. Aerobic exercise and physical activity tuned to the individual's needs will promote good vascular adaptations and facilitate improved recovery following spinal cord injury.
Exercise and physical training have been shown to impact vascular function positively, enhance arterial pressure regulation, and increase stroke volume, collectively contributing to the overall cardiovascular health of individuals with SCI.
Moreover, it is crucial to recognise the impact of physical activity on the mental well-being of individuals with spinal cord injury. However, research has identified a significant correlation between regular physical activity and improved mental health outcomes. By incorporating physical activity into their rehabilitation routine, individuals with SCI can experience alleviation of symptoms associated with depression and anxiety, consequently enhancing their overall quality of life.
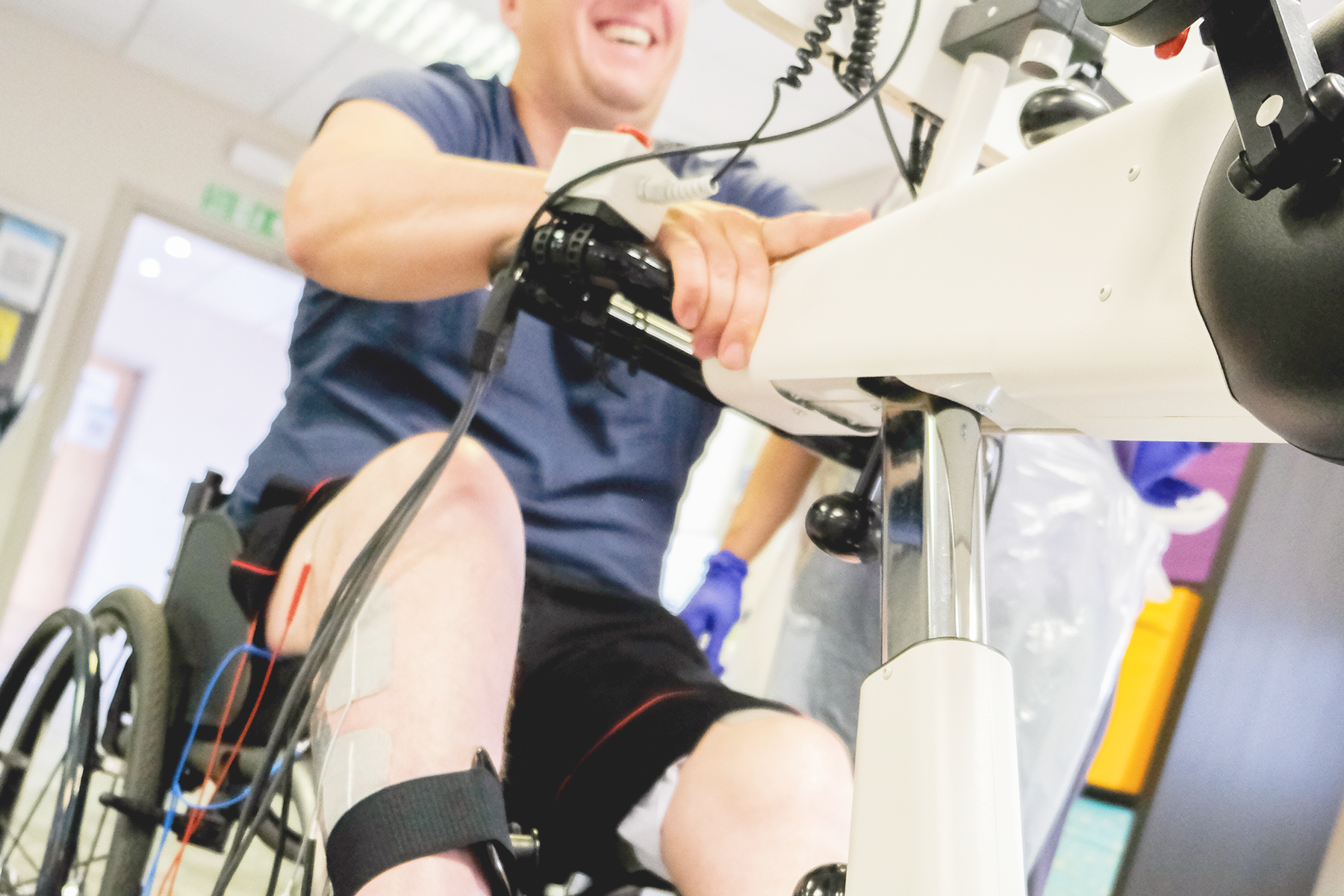
Functional Electrical Stimulation (FES) Cycling can help individuals maintain fitness following a spinal cord injury (SCI). The art of cycling using functional electrical stimulation (FES) of the paralysed leg muscles was first demonstrated during the 1980s. Today, FES Cycling is a well-researched and established technique where electrical currents are applied to paralysed or weakened leg muscles. This causes the muscles to contract in sync with the movement of the bike's pedals. In the case of FES Cycling, these electrical stimuli enable the user's paralysed legs to pedal a bicycle-like device and get active exercise, even when voluntary control over the muscles has been lost due to the SCI.
Benefits of FES Cycling for SCI Individuals:
We have written many articles on this website on the benefits of FES Cycling, and I have summarised what we know below
Improved Cardiovascular Health: Regular FES Cycling can increase heart rate and improve blood circulation, contributing to better cardiovascular endurance and overall heart health. It helps in mimicking the physiological effects of voluntary vigorous exercise, which is often challenging for individuals with SCI.
Enhanced Muscle Strength: By electrically stimulating the leg muscles to pedal a bike against resistance, FES Cycling helps maintain or even increase muscle mass and strength in the lower limbs. This is crucial not just for physical health but also for improving metabolism.
Increased Blood Flow and Oxygen Consumption: FES Cycling promotes increased blood flow to the lower limbs, which is beneficial for skin integrity and can help prevent pressure sores, which is sadly a common issue for individuals with SCI. Enhanced circulation also aids in better oxygen utilisation by the body during exercise.
Prevention of Muscle Atrophy: Regularly engaging in FES Cycling can prevent or reduce the extent of muscle atrophy (wasting away) following SCI due to lack of muscle use. Muscles that are not exercised don't just atrophy; they change their tissue structure.
Improved Venous Return: The rhythmic contraction of the leg muscles during FES Cycling aids in promoting venous return, reducing the risk of deep vein thrombosis (DVT) and other vascular complications associated with SCI.
Enhanced Psychological Well-being: Regular physical activity through FES Cycling can also have significant mental health benefits, including improved mood, greater self-esteem, and a reduced risk of depression, contributing to a better quality of life.
Research and Practical Considerations:
Research supports using FES Cycling to improve cardiovascular fitness in individuals with SCI. Studies have shown improvements in cardiovascular parameters, muscle cross-sectional area, and blood circulation among participants. However, the degree of benefit can vary based on the individual's level of injury, overall health status, and the frequency and intensity of the FES Cycling program. FES Cycling is relatively inefficient at creating muscle contractions, so it should be used diligently and frequently for best results. For optimal results, it's recommended that FES Cycling be part of a comprehensive rehabilitation program tailored to the individual's specific needs and goals.
In the early stages of training, some individuals will demonstrate fairly rapid muscle fatigue due to the changes in muscle fibre type due to the SCI. Persistence is necessary to generate strong and sustainable muscle contractions again.
Muscle activation through Functional Electrical Stimulation (FES) exhibits a distinctive muscle fibre recruitment pattern. At lower levels of stimulation, fast-twitch, fatigueable fibres are preferentially engaged, followed by the recruitment of slow-twitch, fatigue-resistant fibres as the stimulation intensity increases. This recruitment pattern is attributed to the association of fast-twitch fibres with large motor units, which are innervated by large-diameter nerve axons that possess a lower firing threshold when externally stimulated. Consequently, electrically stimulated muscles generally experience rapid fatigue and exhibit limited force generation capacity. Additionally, paralysed muscles initially suffer from poor condition due to disuse atrophy and typically display varying degrees of unwanted contractile activity resulting from heightened reflex responses (spasticity).
Kjaer et al. (1994) conducted a study to compare the performance of eight healthy young males in volitional and FES-induced cycling. Initially, the participants engaged in volitional cycling at a work rate, resulting in an average oxygen uptake of 1.9 litres per minute. Subsequently, complete epidural anaesthesia was administered, causing complete leg paralysis. FES Cycling was then employed to achieve active cycling at a work rate that elicited the same oxygen uptake rate of 1.9 Litres per minute. The findings revealed that during volitional cycling, the average work rate for this oxygen cost was approximately 120 W, whereas, for FES Cycling, it was less than 40 W. This suggests that even in individuals with normal muscle condition, FES-induced cycling is approximately three times less efficient compared to cycling achieved through volitional muscle control.
Thus, considerable scope exists for optimising stimulation patterns to improve cycling performance stimulation patterns.
We suggest that FES Cycling clients start with stimulating the quadriceps and hamstrings. We advise that they strive to make FES Cycling a habit and recognise that after several sessions, they will be able to generate stronger contractions and overcome the apparent fatigue generally present. A programme to include the pre-tibial and gastrocnemius muscles should also be included. Generating active contractions in these muscles increases venous return and helps prevent the common, slow-to-heal skin rubs we often see.
Thijssen et al. (2006) studied the time course of arterial adaptations during six weeks of functional electric stimulation (FES) training and six weeks of detraining in subjects with spinal cord injury (SCI). This was a "before and after"trial with nine subjects who conducted six weeks of twice-weekly FES Cycling sessions followed by six weeks of detraining. Vascular characteristics were measured by plethysmography (baseline and peak blood flow of the thigh) and echo-Doppler (diameter of the femoral artery and flow-mediated dilation [FMD]).
After two weeks of FES training, significant changes were observed in arterial characteristics. These changes included an increase in baseline and peak blood flow, an increase in the diameter of the femoral artery, and a decrease in the femoral artery's FMD (flow-mediated dilation). Upon cessation of training, the baseline and peak thigh blood flow, vascular resistance, and femoral diameter reverted to their pretraining values within one week. However, the FMD of the femoral artery did not fully recover, even after six weeks. Based on these findings, the authors concluded that a two-week hybrid FES training program consisting of four exercise bouts is sufficient to enhance peak leg blood flow, increase arterial diameter, and normalize FMD. Furthermore, detraining leads to a rapid reversal of vascular characteristics within one week.
On the other hand, a study by Jansen et al. (2021) revealed that a 16-week training program consisting of twice-weekly sessions, lasting up to 30 minutes each, on a hybrid bicycle or handcycle did not result in systemic vascular adaptations. The authors propose that a larger sample size and a training protocol involving more frequent and higher intensity sessions, potentially in a home-based setting, with an adapted period before the training, could yield different outcomes. This highlights the challenge of determining the necessary and sufficient exercise dosage.
While there are some approaches that we generally follow with any FES Cycling client, it is important to recognise that each case is different and should be approached accordingly. Many clients use these systems for long-term health benefits, and we must ensure they are easy to use but effective for each individual. The first rule of gaining fitness through exercise is to "stick at it". The research clearly shows cardiovascular function and other benefits from using FES Cycling.
Further Reading
West CR, Alyahya A, Laher I, Krassioukov A. Peripheral vascular function in spinal cord injury: a systematic review. Spinal Cord. 2013 Jan;51(1):10-9. doi: 10.1038/sc.2012.136. Epub 2012 Nov 27. PMID: 23184028.
Hopman MT, Groothuis JT, Flendrie M, Gerrits KH, Houtman S. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol (1985). 2002 Dec;93(6):1966-72. doi: 10.1152/japplphysiol.00897.2001. PMID: 12433934.
Devillard X, Rimaud D, Roche F, Calmels P. Effects of training programs for spinal cord injury. Ann Readapt Med Phys. 2007 Jul;50(6):490-8, 480-9. English, French. doi: 10.1016/j.annrmp.2007.04.013. Epub 2007 Apr 24. PMID: 17482709.
Davis GM, Hamzaid NA, Fornusek C. Cardiorespiratory, metabolic, and biomechanical responses during functional electrical stimulation leg exercise: health and fitness benefits. Artif Organs. 2008 Aug;32(8):625-9. doi: 10.1111/j.1525-1594.2008.00622.x. PMID: 18782133.
Hettinga DM, Andrews BJ. Oxygen consumption during functional electrical stimulation-assisted exercise in persons with spinal cord injury: implications for fitness and health. Sports Med. 2008;38(10):825-38. doi: 10.2165/00007256-200838100-00003. PMID: 18803435.
Ter Woerds W, De Groot PCE, Van Kuppevelt DHJM, Hopman MTE. Passive leg movements and passive cycling do not alter arterial leg blood flow in individuals with spinal cord injury. Physical Therapy, 86(5): 636-645, 2006.
Dick H. Thijssen, Reinier Ellenkamp, Paul Smits, Maria T. Hopman. Rapid Vascular Adaptations to Training and Detraining in Persons With Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. Volume 87, Issue 4, 2006, Pages 474-481. ISSN 0003-9993. https://doi.org/10.1016/j.apmr.2005.11.005.(https://www.sciencedirect.com/science/article/pii/S0003999305013833)
Jansen, E., de Groot, S., Smit, C.A. et al. Vascular adaptations in nonstimulated areas during hybrid cycling or handcycling in people with a spinal cord injury: a pilot study of 10 cases. Spinal Cord Ser Cases 7, 54 (2021). https://doi.org/10.1038/s41394-021-00417-2
Williams AM, Ma JK, Martin Ginis KA, West CR. Effects of a Tailored Physical Activity Intervention on Cardiovascular Structure and Function in Individuals With Spinal Cord Injury. Neurorehabilitation and Neural Repair. 2021;35(8):692-703. doi:10.1177/15459683211017504
Hoekstra F, McBride CB, Borisoff J, et al. Translating the international scientific spinal cord injury exercise guidelines into community and clinical practice guidelines: a Canadian evidence-informed resource. Spinal Cord. 2020;58:647-657.
Kenneth James Hunt. Control Systems for Function Restoration, Exercise, Fitness and Health in Spinal Cord Injury. A thesis submitted for the degree of Doctor of Science in Engineering (DSc (Eng)) at the University of Glasgow in January 2005
M. Kjaer, G. Perko, N. H. Secher, R. Boushel, N. Beyer, S. Pollack, A. Horn, A. Fernandes, T. Mohr, S. F. Lewis, and H. Galbo, Cardiovascular and ventilatory responses to electrically induced cycling with complete epidural anaesthesia in humans, Acta Physiol. Scand., vol. 151, pp. 199–207, 1994.
Maria T. E. Hopman,Jan T. Groothuis,Marcel Flendrie,Karin H. L. Gerrits, and Sibrand Houtman. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. Journal of Applied Physiology 2002 93:6, 1966-1972
Hooker, S.P.; Figoni, S.F.; Rodgers, M.M.; Glaser, R.M.; Mathews, T;. Suryaprasad, A.G. & Gupta, S.C. (1992), 'Physiologic effects of electrical stimulation leg cycle exercise training in spinal cord injured persons., Arch Phys Med Rehabil 73(5), 470--476.